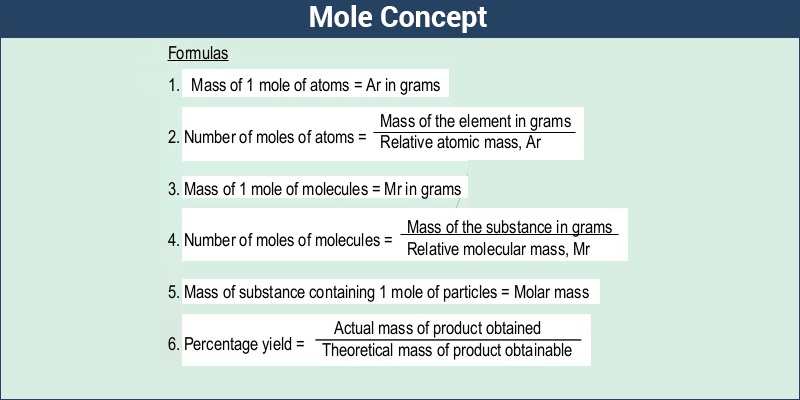
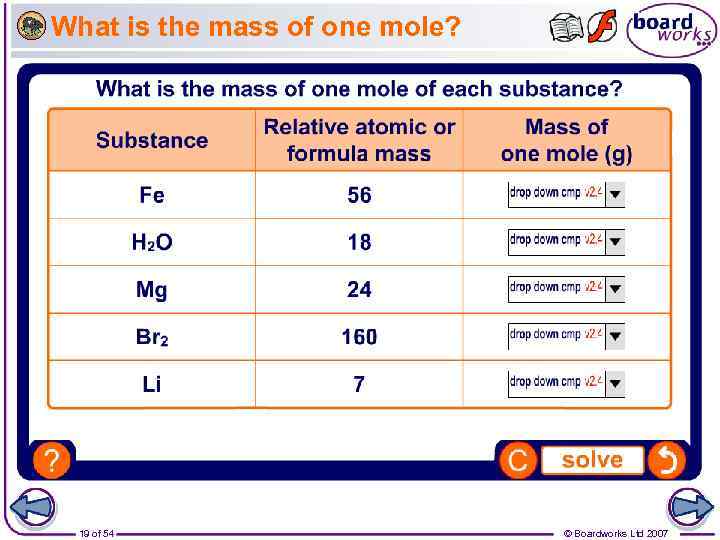
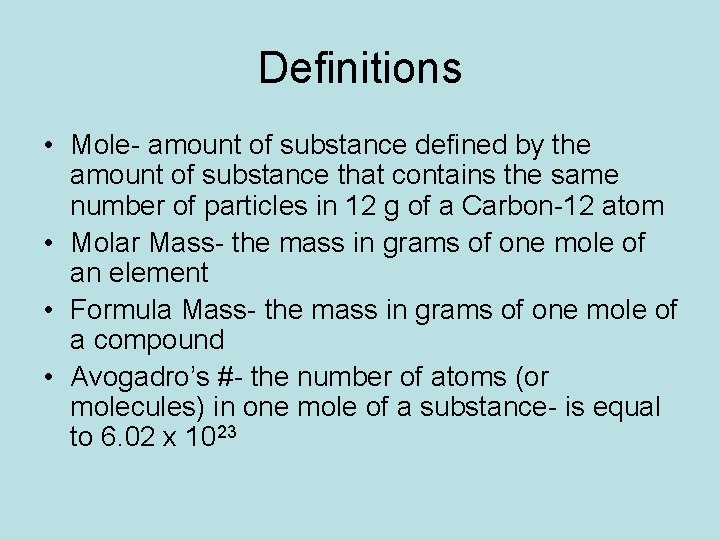
How do you calculate the moles of a substance?
The mole is the amount of a substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12; its symbol is 'mol." When the mole is used, the elementary entities must be specified and may be atoms. One mole is the Avogadro number of particles (atoms, molecules, ions or electrons) in a substance.
1 Answer
In order to calculate the moles of a substance, you need to know the mass of the substance and its molar mass. Molar mass is the atomic weight in grams/mol.
One Mole Of A Substance Is Equal To
Example:
How many moles of copper(II) sulfate,
Molar Mass of
Subscript x molar mass =
1 x 63.456g/mol Cu = 63.456g/mol Cu
1 x 32.065g/mol S = 32.065g/mol S
4 x 15.999g/mol O = 63.996g/mol O
Total: 159.517g/mol
1mol

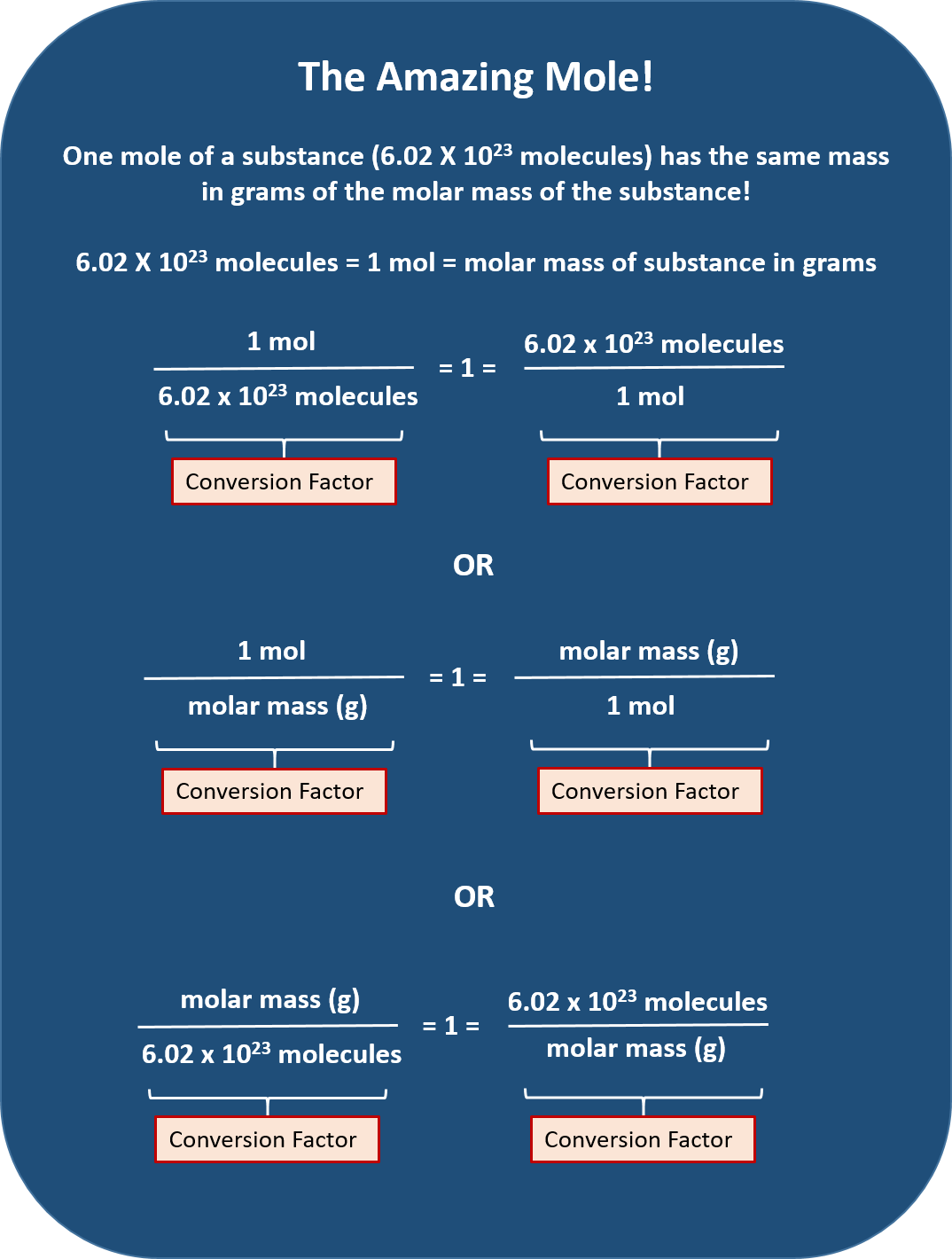
One Mole Of A Substance
Now multiply the known mass x the conversion factor with moles on top and grams on bottom. This will cancel the grams and leave the moles.
One Mole Of A Substance Contains
Related questions