[Download the accompanying PDF worksheet.]
Calculate the Average Atomic Mass
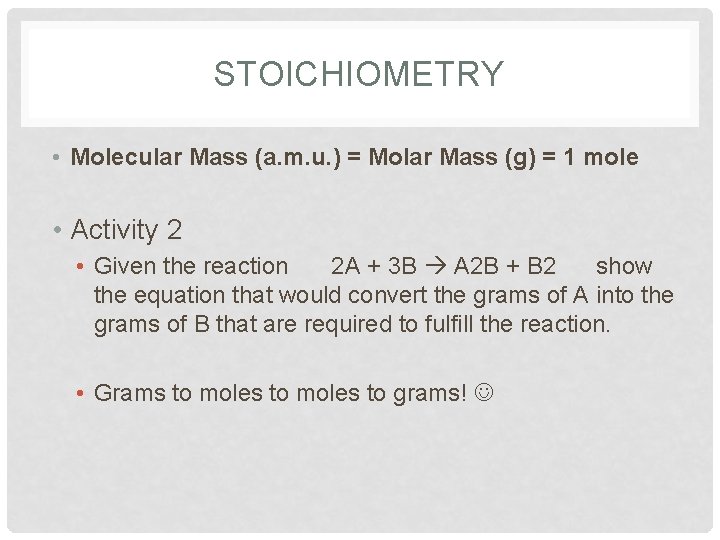
- Calculate the average atomic mass of an element with two naturally occurring isotopes: 85X (72.15%, 84.9118 amu) and 87X (27.85%, 86.9092 amu). What is this element?
- Chromium has the following isotopic masses and relative abundances. Calculate the average atomic mass of chromium to two decimal places.
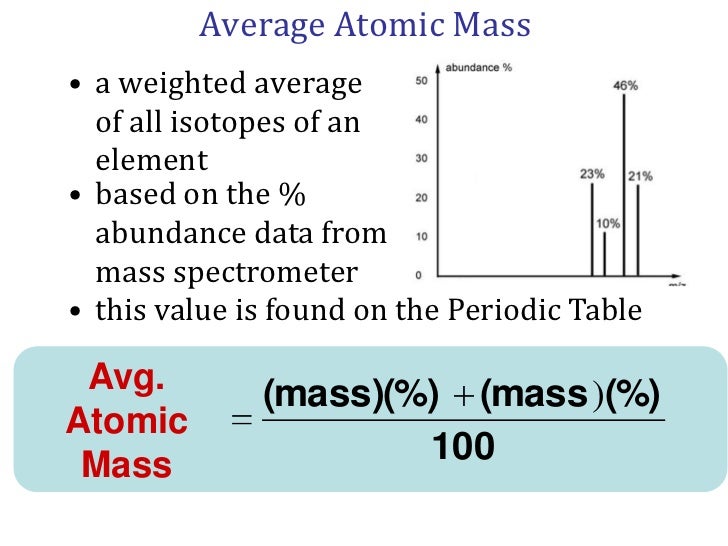
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. According to the International Union of Pure and Applied Chemistry (IUPAC), 1 atomic mass unit is defined as 1/12 the mass of carbon-12. Therefore, carbon-12 has a mass of 12 amu. Carbon-12 is the. An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. For example, one atom of helium-4 has a mass of 4.0026 amu. An atom of sulfur-32 has a mass of 31.972 amu. Chemistry Building Exit Form. Research Continuity website. C–H bond breakers seek smarter tools. Upcoming Events. April 19, 2021 Inorganic Seminar Professor Oliver Wenger University of Basel April 20, 2021 Physical Seminar, Literature Saba Kanwal. An atomic unit of mass is defined as accurately 1/12 the mass of a carbon-12 atom. The carbon-12 atom has six neutrons and six protons in its nucleus. The atomic unit mass is symbolized as AMU or amu. 1 AMU = Average of the proton rest mass and the neutron rest mass. 1 AMU = 1.67377 x 10 -27 kilogram or 1.67377 x 10 -24 gram.
| Mass Number | Iostopic Mass (amu) | Percent Abundance |
| 50 | 49.9461 | 4.35% |
| 52 | 51.9405 | 83.79% |
| 53 | 52.9407 | 9.50% |
| 54 | 53.9389 | 2.36% |
[Want more practice problems? Check out Atomic Mass Practice Problems Part II.]
[View the accompanying Lesson on Names & Formulas of Acids here.][Download ..
[View the accompanying Lesson on Names & Formulas of Molecular ..
Related Posts:
Comments
comments
Method of Assessment
There will be two (2) tests in the course, a midterm and a final given in modules four and eight respectively. The tests in this class will consist of multiple choice questions and/or short answer problems. Tests will be three hours long and focus on the material covered in the course. Tests may be administered using the Examity test proctoring service. Please verify in the course announcements and/or the Lessons tab if the course will use test proctoring. It is the student’s responsibility to notify the instructor and the test proctoring service regarding any schedule changes or non- disability related accommodations. The course will have a comprehensive paper that will measure student understanding of the course objectives along with researhc and writting skills. Please refer to the APA Manual 6th edition for the format of the paper.
B.Assignments:
Course assignments will be given using multimedia software (e.g. MyLabsPlus, Connect, etc.). These assignments will be a series of quizzes consisting of exercises, problems, and simulations. Assignments will have a specific due date with specific instructions. Late assignments will be subject to the university’s Late Work/Make-up Policy detailed in the student handbook. Please be advised the instructor reserves the right to implement their own late assignment policy.
C.Homework:

Throughout the term, homework will be given in several modules to test student understanding of the material. Homework will be given using multimedia software (e.g. MyLabsPlus, Connect, etc.) and consist of questions, problems, or simlations. Please be advised the instructor reserves the right to implement their own late assignment policy.
D.Discussion Forums:
Participation in the discussion forums is an essential component of the final grade. All students are expected to engage in lively discussions and answer instructor follow-up questions. The quality of participations along with student netiquette will be a part of the grade.
Assessment of the Course Objectives
Course Objective(s) | Assessment Method(s) |
1 - 6 | Test question, paper, assignment, discussion, and homework |
The following distribution will be used in assigning grades (decimal points will be rounded to the nearest whole number at semester’s end).
Grade | Quality Points/Grading Percent |
A | 4.0/ 100 – 94 |
A- | 3.67/ 93 – 90 |
B+ | 3.33/ 89 – 87 |
B | 3.0/ 86 – 84 |
B- | 2.67/ 83 –80 |
C+ | 2.33/ 79 – 77 | |
C | 2.0/ 76 – 73 | |
C- | 1.67/ 72 – 70 | |
D+ | 1.33/ 69 – 67 | |
D | 1.0/ 66 – 64 | |
D- | 0.67/ 63 – 60 | |
F |
|
Amu Chemistry
| Name | Grade % |
|---|---|
| Discussion Forums | 10.00 % |
| Week 4 Forum | 5.00 % |
| Week 8 Forum | 5.00 % |
| Homework | 20.00 % |
| Chapter One Homework | 3.33 % |
| Chapter Two Homework | 3.33 % |
| Chapter Three Homework | 3.33 % |
| Chapter Four Homework | 3.33 % |
| Chapter Five Homework | 3.33 % |
| Chapter Six Homework | 3.33 % |
| Quizzes | 30.00 % |
| Chapter One Quiz | 5.00 % |
| Chapter Two Quiz | 5.00 % |
| Chapter Three Quiz | 5.00 % |
| Chapter Four Quiz | 5.00 % |
| Chapter Five Quiz | 5.00 % |
| Chapter Six Quiz | 5.00 % |
| Final Assessment | 10.00 % |
| Week 8 Paper | 10.00 % |
| Tests | 30.00 % |
| Midterm | 15.00 % |
| Final | 15.00 % |
